Visit to Core Resources lab
MIWATCH Research Officer Sibele Nascimento shares her recent visit to Core Resources' lab and observations on her flotation experiment.
In March I had the pleasure of visiting Core Resources which is a specialist metallurgical laboratory and process engineering group. In our project, we are characterising mineralogically complex tailings from Tasmania and testing the potential for cobalt extraction. One part of this work is to conduct flotation and leaching experiments which is what we are performing with the Core Resources team. The testwork is essential to establish if cobalt extraction could be a technically feasible rehabilitation option for the site.
These tests I observed (Test A and Test B) at the Core Resources labs were the first flotation experiments on these particular tailings. Therefore, the main goal here was not to obtain a high grade and/or high recovery concentrate, but to understand how pyrite would behave with the reagents tested and what adjustments would be required in the next flotation steps.

- Test A was divided into 4 stages and performed at pH 8. In the 1st, 2nd and 3rd stages, potassium amyl xanthate (PAX) was added as a strong collector to concentrate pyrite. In 3rd and 4th stages, a few drops of methyl isobutyl carbinol (MIBC) frother was added to stabilize the froth.
- Test B was also divided into 4 stages with pH once again kept to 8. The difference here was that an activator was used before the collector and frother. Copper sulphate (CuSO4) was the activator added in the 1st stage. PAX was added in the 1st, 2nd and 3rd stages. The MIBC frother was incorporated in the 3rd and 4th stages.
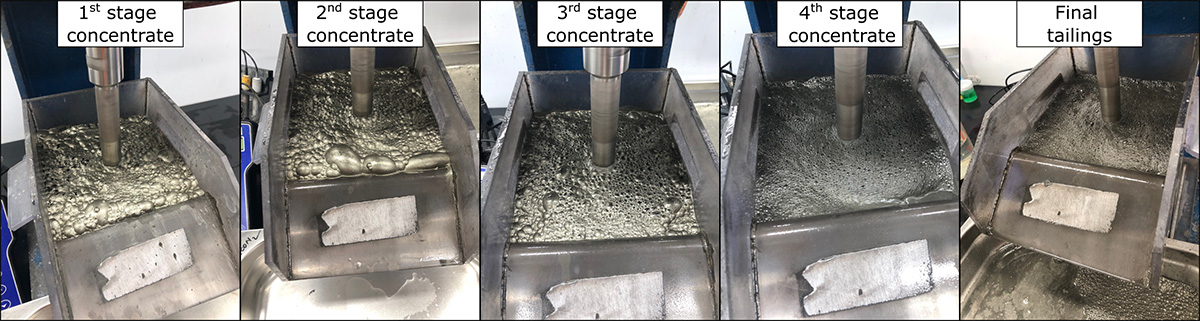
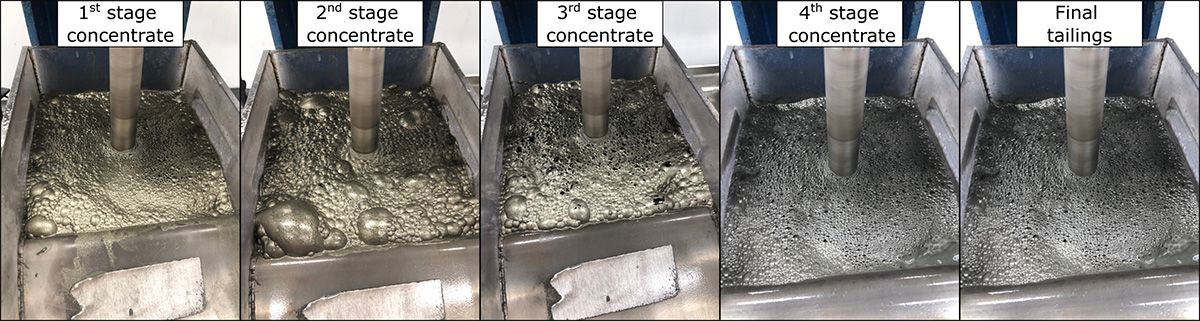
Whilst chemical assay and mineralogical results are pending, some conclusions can be drawn based on visual observation of the concentrate.
- In Test A, the 1st stage flotation produced a high pyrite concentrate as indicated by the golden colour, whereas in stages 2, 3, and 4 pyrite concentrations are likely to have dropped considerably.
- In Test B, the distinct pyrite colour was observed in patches, indicating that the pyrite concentration was well distributed across concentrates produced at each stage.

This visit gave me a great opportunity to understand how to design my own flotation experiments on mine tailings sourced from our Queensland tailings sites. One of the Core Resources experts defined flotation as an “art” because each person has their own ability of doing it and no experiment will be exactly like the other. Samples could be seen as our canvas, reagents our ink and the concentrates the final art. As I love these types of analogies, I couldn’t agree more.

I left their labs extremely excited and inspired to do my own flotation experiments and my own pieces of “art”, with support from colleagues at the JKMRC Flotation Chemistry team. Stay tuned for updates on our tailings flotation experiments in the coming months.
