My first authored PhD journal article – A review of indium cycling from ore to mine waste
This month, I received exciting news about the publication of my first-authored paper! As part of my PhD journey, and being a non-native English speaker, one of the initial challenges I faced was writing a review article. In this paper, my supervisors and I delved into the current state of knowledge regarding the cycling of indium from ore to mine waste deposits, exploring the potential for its recovery.

After a year and a half of writing, revising, and refining, I have some reflections on my personal thoughts as a PhD student navigating the process of writing a first review journal article. Here are 10 steps I'd like to share that were helpful to me on this journey:
- Seek inspiration from other papers.
- Develop and draft an outline for your article.
- Organize the papers that you read and select, aligning them with your outline (using a reference management software can be helpful).
- Do not wait for inspiration to strike; keep writing consistently.
- Identify when and how your focus on writing is optimal.
- Embrace feedback from your supervisors, as they continually strive to enhance both your article and your skills.
- Convert your knowledge into figures and diagrams, fostering creativity while enabling your audience to follow your ideas. In addition, it is a good exercise to digest the tons of articles that you will need to read to write a review. Although this may require learning new software during the writing process, it will greatly enhance your article and future skills.
- As a review article, it is crucial to exercise restraint and refrain from continuously adding more data. Otherwise, the article runs the risk of becoming an endless journey.
- If possible, enlist the help of a trusted friend to provide fresh eyes.
- Print and proofread your article.
Now, let's delve into the findings. Indium, a critical metal with a crucial function in low-carbon technology, has gained worldwide attention due to its vulnerability to supply disruptions. While it can be found in various primary and secondary deposits, there is still much to learn about the geochemistry, mineralogy, and cycling of indium, especially in mine waste environments.
The highest indium content has been commonly described in Cu-rich parts of polymetallic deposits related to magmatic-hydrothermal sources, in contrast with lower indium content in deposits formed at low temperatures. Through an extensive review of published data (n=411), we have identified 45 minerals containing indium. Sphalerite is the main carrier of indium, often incorporating it through a process called couple substitution, involving more elements such us copper, zinc, silver, germanium, gallium, and tin. Magmatic-hydrothermal sphalerite tends to be characterised by higher In, Cu, Sn, and Fe, and lower Ge contents and is associated with chalcopyrite, stannite, kësterite, cassiterite, and magnetite. Specifically, indium-rich minerals are found within xenothermal/epithermal veins and mantos associated with tin systems, as opposed to those found in copper systems.
When these minerals break down, leads to the accumulation of indium in water and sediments. Additionally, atmospheric fallout from smelting processes contributes to the deposition of indium. In fact, our calculations suggest that solid mine waste can contain indium concentrations up to 10,000 times higher than the average crustal abundance, while aqueous mine waste can be as much as 55,000 times higher.
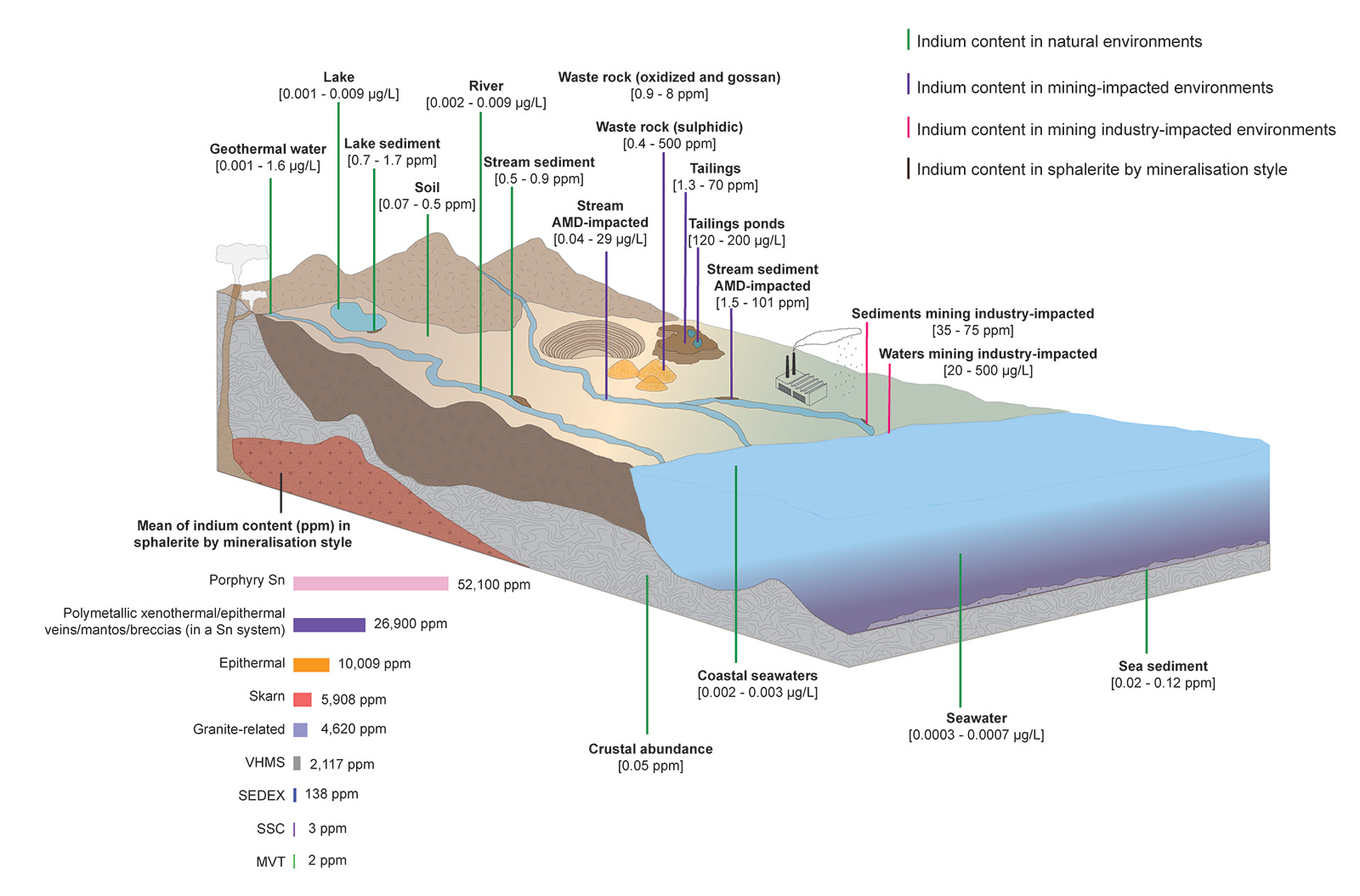
In aqueous systems, indium exists as a free and mobile ion (In3+) at acidic pH and at 25°C. However, at pH > 4, indium can form hydroxide complexes, which are believed to play a crucial role in controlling its transportation. Various mechanisms such as adsorption and coprecipitation have been proposed for the occurrence of indium-rich secondary minerals, although limited research has been conducted on these types of mineral phases.
To recover indium from sulphidic mining waste, bioleaching has emerged as a promising environmentally friendly and efficient option. Meanwhile, for extracting indium from smelting residues, methodologies such as leaching and selective extraction using deep eutectic solvents have been implemented. Additionally, new technologies like phytomining (using plants to extract metals) and solvometallurgy (non-aqueous solutions to extract metals) have been tested for their potential in indium recovery.
The significance of indium in low-carbon technology and its susceptibility to supply disruptions make it a critical metal deserving of attention. Understanding its behaviour from ore to mine waste deposits and exploring innovative recovery methods are crucial for sustainable resource management. With continued research efforts, we can unlock the full potential of indium and contribute to a greener future.
This research project is supported by the Geological Survey of Queensland (GSQ) under the New Economy Minerals Initiative (NEMI) program. Additionally, I would like to thank everyone who took time to read, give advice, and teach skills that were included in this article.
Access the paper – doi.org/10.1016/j.gexplo.2023.107312

