Copper is a powerhouse metal, driving everything from data centres to solar panels and other green energy innovations. But getting it out of the ground—especially from tough minerals like chalcopyrite (CuFeS₂)—is no easy feat. This blog dives into a recent study that tackles these challenges, offering hope for a more efficient and eco-friendly copper supply. Whether you’re a scientist or just curious about the future of mining, here’s what you need to know!
The Copper Puzzle
Extracting copper from its natural sulfide forms is tricky because of something called "passivation." Imagine the copper mineral getting coated with a stubborn layer—like a shield—that blocks the chemicals (called reagents) needed to pull the copper out. These layers can include things like elemental sulfur, secondary copper sulfides (think covellite, CuS), iron hydroxide (FeOH3), or jarosite (KFe₃(SO₄)₂(OH)₆). Together, they inhibit the process, slowing down copper extraction and making it less efficient.
What the Study Dug Into
The research, titled "Coupled Dissolution with Reprecipitation (CDR) Reactions and their Impact on Copper Sulphide Mineral Surface Area and Dissolution Rates," explored how these coating layers form and how to break through them. The team tested different solutions—called lixiviants—on copper sulfide minerals like chalcopyrite and bornite. These included:
- FeCl₃-only (iron chloride)
- AlCl₃-rich (aluminum chloride)
- NaCl-rich (sodium chloride, aka salt)
- CaCl₂-rich (calcium chloride)
They wanted to see how these solutions change the mineral surfaces and influence copper release.
What They Discovered
Here’s the exciting part:
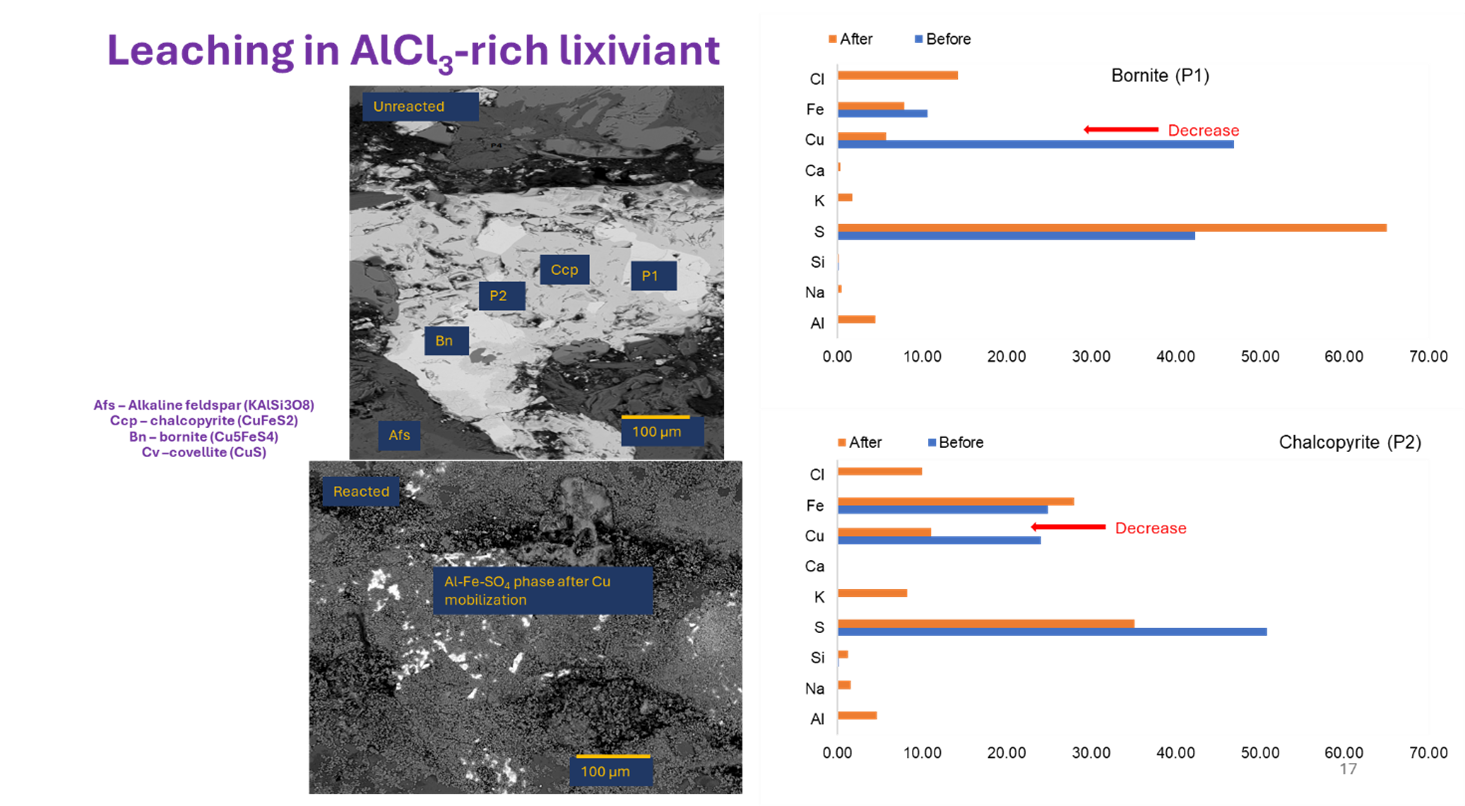
- Aluminum chloride (AlCl₃) shines. This solution worked wonders on both chalcopyrite (CuFeS2) and bornite (Cu5FeS4). It kept iron from forming pesky compounds that jam the surface and used a clever ion swap (Al³⁺ for Fe³⁺) to keep the copper-releasing process humming.
- A special layer forms. Under consitent acidic and potential conditons, without adding external acid, an aluminum-sulfate phase formed, which helped maintain the right chemistry for copper to dissolve more easily.
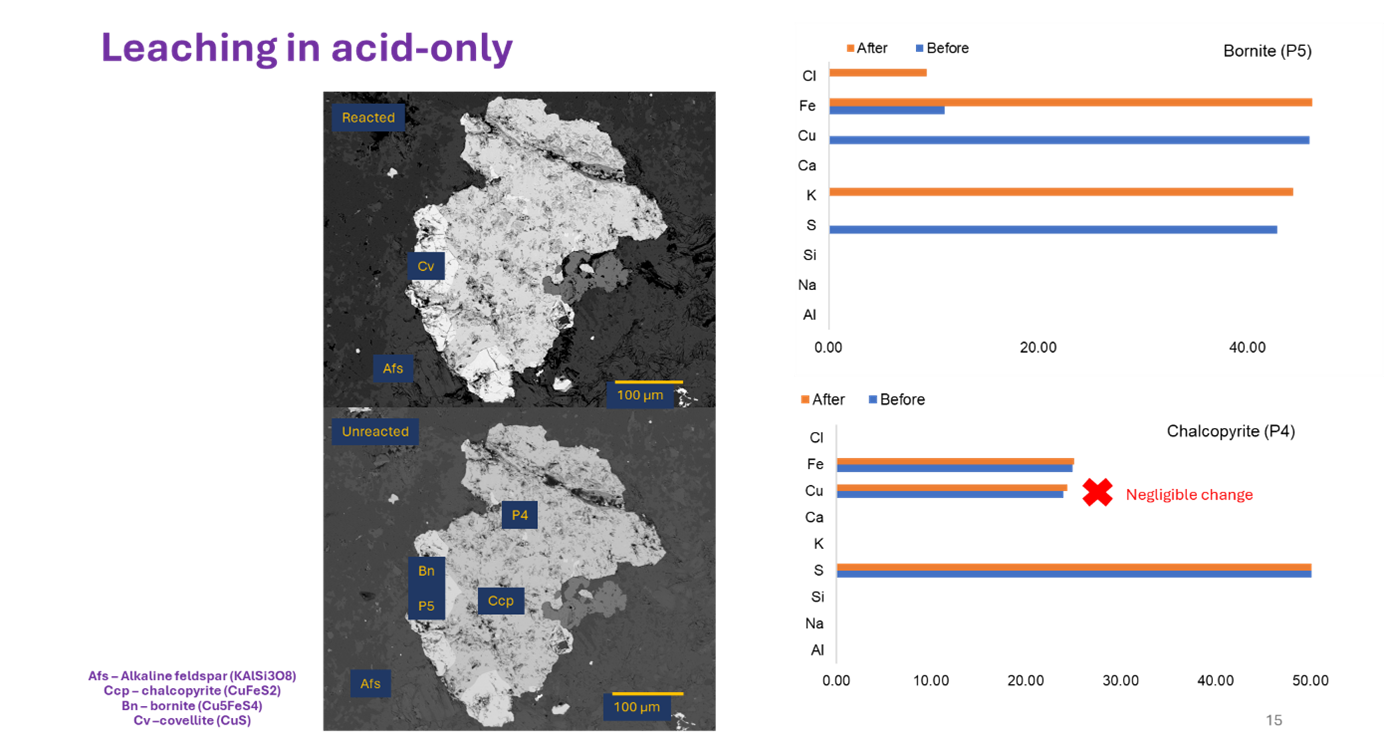
- Other solutions were pickier. The FeCl₃-only, NaCl-rich, and CaCl₂-rich solutions only worked on bornite, leaving chalcopyrite coated with leftover copper-sulfur and iron-sulfate layers.
- Strength matters. The more concentrated the solution (its "ionic strength"), the more copper released.
Why This Is a Big Deal
This study sheds light on how and what impedes copper extraction—and how we might tackle this. By using solutions with multivalent ions like aluminum (Al³⁺), we could boost copper recovery from coarse materials while reducing waste and energy. It’s a step toward greener mining that could one day be tested in real-world mines. While it’ll take time to scale up these ideas, the groundwork is laid for efficient, more sustainable ways to meet our copper needs.
What’s Next?
The future looks promising! The researchers intend to:
- Test these solutions for field trials to see if they work on a larger scale.
- Crunch the numbers to figure out if they’re socio-economicallycost-effective.
- Explore how these methods might help extract other metals like gold or cobalt.
By tackling passivation head-on, we’re opening doors to a cleaner, more efficient copper supply—one that powers our tech-driven, green-energy world without overburdening the planet.
What do you think—could this be the key to unlocking copper for the future?
Read more here:
Website: https://www.mdpi.com/2075-163X/15/3/214
PDF Version: https://www.mdpi.com/2075-163X/15/3/214/pdf