Herbarium Resources
About the Centre for Mined Land Rehabilitation Herbarium
What is the herbarium?
The herbarium is located on level 5, Sir James Foots Building and is a dedicated room with a large collection of plant specimens and plant identification resources such as powerful microscopes and a library of identification books. The herbarium contains up to 5,000 specimens as well an online digital herbarium which is a database containing high resolution scans of over 1,200 plant specimens.
What makes the herbarium unique?
The herbarium is the only dedicated herbarium on campus and is a unique resource for researchers and students to confirm the identification of plant species related to land rehabilitation in Queensland, New South Wales and the Northern Territory. The herbarium also has one of the most comprehensive collections of species that are found in the endangered swamps of the Newnes Plateau (Western Blue Mountains region, NSW). The room contains an imaging microscope (Leica M205A) capable of high resolution magnification (160x), digital display, image capture, live measurements and multi-focus image stacking. The room is set at constant temperature (20°C) and humidity (45%) to maintain the preservation of the samples.
How does the herbarium service other parts of the University and industry?
Accurate plant identification is fundamental aspect of environmental surveys, particularly when studying the impacts of mining and the success of land rehabilitation. The herbarium is a place that researchers and students from around the university can work on plant identification, mount specimens for storage, or capture high resolution imagery of plant samples.
Access to the digital herbarium is available through the CMLR home page and includes high resolution images of scanned specimens, maps of collections and links to online resources. This digital herbarium is highly valuable to the mining industry, land managers, environmental scientists and plant enthusiasts, with a particular focus around the theme of land rehabilitation.
Contact: Phill McKenna: p.mckenna@cmlr.uq.edu.au or Vanessa Glenn: v.glenn@cmlr.uq.edu
Plant pressing and mounting equipment
Below is a selection of some of the most regularly used plant pressing and mounting equipment with supplier’s details.
 Plant presses
Plant presses
Herbarium Supply Co. (USA)
 Clear archival tape
Clear archival tape
3M™ Polyester Film Tape 850 Transparent 0.5
Special order from Archival Survival
http://www.archivalsurvival.com.au/
 Polyurethane Foam
Polyurethane Foam
(for bulky plant pressing)
Herbarium Supply Co. (USA)
http://herbariumsupply.com/
Clarke Rubber (Aust.) – cut to size
 Gummed Paper Tape
Gummed Paper Tape
(for mounting):
Paper Hinging Tape (Lineco) L533-0751
Archival Survival
http://www.archivalsurvival.com.au/
 Jeweller's tags or strung tags
Jeweller's tags or strung tags
Archival Survival
http://www.archivalsurvival.com.au/
University Products (USA)
http://www.universityproducts.com/
 Mounting card – typically 42 x 27cm (A3)
Mounting card – typically 42 x 27cm (A3)
Archival Survival
http://www.archivalsurvival.com.au/
University Products (USA)
http://www.universityproducts.com/
or standard stationary suppliers with at least 280-300gsm as this now has life span of >100 years.
 Herbarium boxes
Herbarium boxes
Custom-made from Archival Survival
http://www.archivalsurvival.com.au/
University Products (USA)
http://www.universityproducts.com/
 Dissecting Kit
Dissecting Kit
Australian Entomological Supplies Pty. Ltd.
http://www.entosupplies.com.au/
CMLR Herbarium guidance documents
Through the upgrade of the herbarium which commenced in 2012, a series of internal manuals and guidance documents were developed. The aim of these documents was:
- To provide an outline of the plant specimen collection management systems
- To provide step-by-step manuals for operating equipment such as microscopes and digitising copystand
- To ensure that specimens for inclusion into the CMLR Herbarium are of the highest quality and identification possible
- To highlight all of the upgrades
Whilst the actual manuals are not made available online, key extracts are provided in the links for the Additional Resources The cover of each of the manuals is shown below:
 Plant Collections Manual
Plant Collections Manual
By Nic McCaffrey, Cameron Kilgour, John Van Osta & CMLR Herbarium Committee, 2013
 Specimen Digitising Manual
Specimen Digitising Manual
By John Van Osta & Nic McCaffrey, 2013
 General requirements for inclusion into CMLR Herbarium
General requirements for inclusion into CMLR Herbarium
By Ashley Lawson, 2013.
 Leica M205A Microscope Manual
Leica M205A Microscope Manual
By Hao Ran Lai & Nic McCaffrey, 2013
Acknowledgements:
The upgrade and specimen processing work would not have been possible without the help from many research and admin staff at CMLR, SMI and the team at SilverBiology. Particular thanks go to summer students and volunteers John van Osta, Jessica Cooke, Ashley Lawson, Hao Ran Lai, Rossiti Karim, Huong Nguyen and Lucy Gramenz.
Creating Herbarium specimen labels using mail-merge
This is a simple guide for creating specimen labels using Microsoft Word to produce a batch-processed, professional looking label. This assumes that you have a place where raw data are kept, such as on a spreadsheet (eg. Microsoft Excel).
Steps:
- You will need to create a new template by using “step by step mail merge wizard” in Microsoft Word or alternatively search for it in your web browser.
- To prepare the data for the mail merge template, your data will need to be arranged in columns with the top row as the column header.
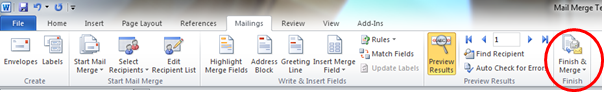
- With the label template open, click “select recipients” in the “Mailings” tab.
- Click “Use Existing List”, then navigate to and open the appropriate database file (eg. spreadsheet containing specimen data)
- Click “Preview Results” in the Mailings tab to verify whether the data has been incorporated into the specimen label template correctly.
- Click “Finish & Merge” then “Edit Individual Documents”. The “Merge to New Document Window” should open
- If you want to create labels for all data series in the database (e.g. each row in excel) select “All” and then select “OK”
- If you want to create a single label for that page that is currently previewed select “Current record”
- If you want to create labels for only some of the data series in a database (e.g only last 100 rows in excel) select “From” and specify which rows
- A new document should open with your completed labels
- Print labels 4 pages per sheet, one sided.
An example of an output of the specimen label is shown below. Bold type is from the Word template and normal font was derived from Excel.

Making barcodes using cheap labelling machine
Barcodes are often used as a primary identifier linking physical specimens, records within a collection database and sometimes specimen images. Due to the important role of this identifier, barcode information is usually derived from a predetermined alphanumeric character then read from the specimen sheet using a barcode reader to avoid transcription errors that occur when keying numbers by hand.
To create a Global Unique Identifiers (GUID), we used our institution code (recommended in Darwin Core) and a sequential number. Sequential numbers are less time consuming and have a lower likelihood of accidentally giving two or more specimens the same number, which can happen if using a matching accession number or collector code.
Barcode example:

Details for CMLR barcode:
- Name: The University of Queensland
- Unique collection code = UQCMLR
- Sequential numbers without repetition from 000001 – 999999
- Example: UQCMLR000001
- 6 digits were used.
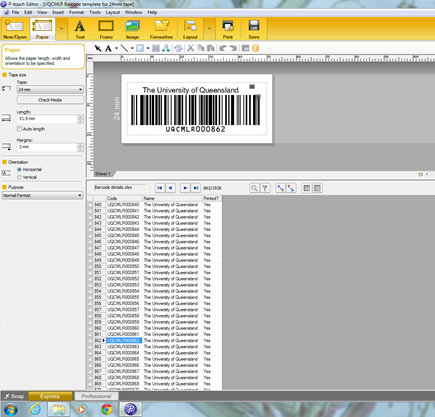
We used a Brother P-touch but there are many cheap labelling machines which can be connected to a computer to make barcodes. The tape lasts for many years, making it suitable for long-term
- Create a spreadsheet with your unique identifiers – this will be loaded into the labelling software.
- We used Code 128, an alphanumeric or numeric-only barcode standard as the basis but there are many to choose from to suit your purpose.
- Using the ‘wizard’ in the software, we connected the spreadsheet, chose the barcode and based it on 24mm tape. 12-18mm tape works but limits readability of the text above and below the barcode area.
- You can print the desired amount of barcodes needed as you go.
- Once the barcodes have been printed go back into the excel spreadsheet and fill in ‘yes’ in the ‘Printed column’ for those barcodes printed.
An example of the barcode loaded in the software and connected spreadsheet are found below:
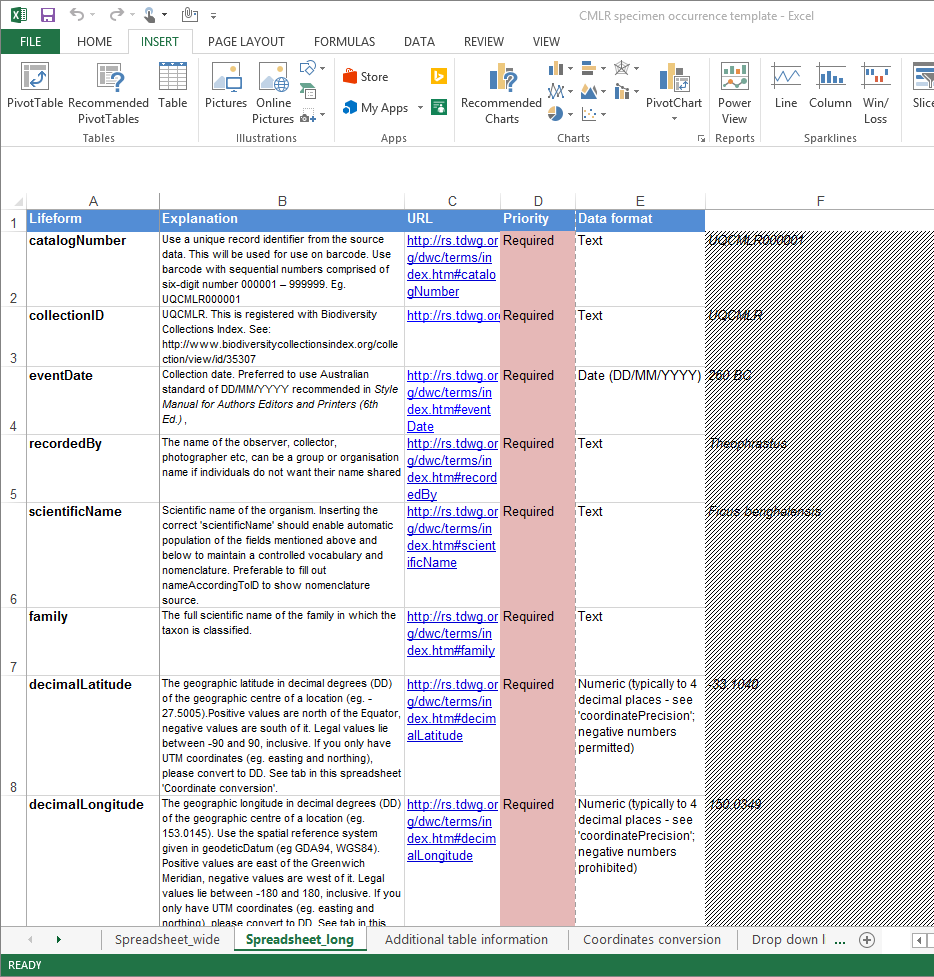
Developing a specimen collection spreadsheet
We developed a specimen collection and occurrence spreadsheet based on Darwin Core international standards and Atlas of Living Australia’s ALA occurrence data template.
Click here to download the spreadsheet.